Bromine Water Test Equation
The bromine is decolourised because a. This allows us to tell alkenes apart from alkanes using a simple chemical test.

Test For Unsaturation 3 4 4 Aqa As Chemistry Revision Notes 2016 Save My Exams
Mild oxidation of glucose with bromine water gives gluconic acid.
. In organic chemistry the bromine test is a qualitative test for the presence of unsaturation carbon-to-carbon double or triple bonds phenols and anilines. Label the test tubes 1-6. The presence of unsaturation in a fatty acid or a triacylglycerol can be detected by reaction with bromine.
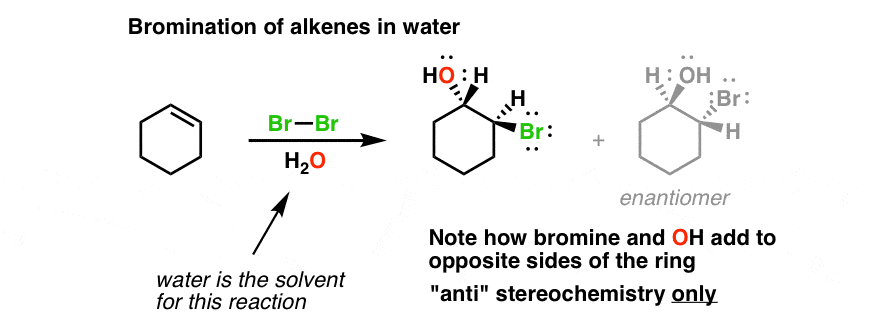
Bromine water is just a yellow mixed solution with a strong oxidation ability due to the combination of diatomic bromine Br2 and water H2O. Bromine water commonly known as bromide bromate solution is a chemical compound having the formula Br2. The double bond can be written as carbon carbon double bond or as CC.
The bromine water test is a qualitative test used to distinguish alkenes and alkynes from alkanes. Bromine Water Test Functional Groups. Find an answer to your question equation of bromine water test nirmla5p nirmla5p 28122019 Science Secondary School answered Equation of bromine water test 2 See answers.
Trying to help you to learn Chemistry online. How does the bromine water test work. Notice the multiple substitution around the ring - into all the activated positions.
Alkene groups react with bromine water in the dark condition and undergo an additional reaction to give a decolourised solution. Alkenes are generally more desirable than alkanes as they are more reactive due. 1-butanol 2-butanol tert-butyl alcohol phenol decene Procedure Set up 6 small 12 X 75mm test tubes in a test tube rack in the hood.
The precipitate is 246-tribromophenylamine. Add 10 mg of a solid unknownknown or 100 l of a liquid unknown to tube 1. The slideshow shows this process.
The reaction with bromine water. Rgure 1071 The bromine water test for unsaturated hydrocarbonsThe unsaturated hydrocarbon decolorizes. Hydrogen sulfide may interfere owing to sulfur formation and must be boiled out firstThe solution to be tested must be acid with acetic acid.
Glucose reacts with hydroxylamine to form a monoxime or adds only one mole of HCN to give a cyanohydrin. Bromine water is an orange solution of bromine. An unknown sample is treated with a small amount of elemental bromine in an organic solvent being as dichloromethane or carbon tetrachloridePresence of unsaturation andor phenol or aniline in the sample is shown.
Alkane does not react with the bromine water solution and the brown-red or orange colour of bromine solution. This is exactly like the reaction which happens with phenol. If the orange color of a bromine solution added to a lipid fades quickly an addition reaction has occurred and the oil or fat is unsaturated.
Organic alkenes - Test with bromine water. Conduct qualitative investigations to test for the presence in organic. One has an alkane solution and the other one has an alkene solution.
Bromine water test is a chemical test to differentiate between alkenes and alkanes. The presence of the CC double bond allows alkenes to react in ways that alkanes cannot. The reaction between bromine and alkenes is an example of a type of reaction called an addition reaction.
Alkenes can decolourise bromine water but alkanes cannot. It becomes colourless when it is shaken with an alkene. The general formula for alkenes is.
Alkenes are a homologous series of hydrocarbon compounds with at least one double bond between two of the carbon atoms on the chain. There are 2 test tubes with aluminium foil wrapped around and they are put in the dark. This reaction indicates the presence of either an aldehyde -CHO or a ketone C O group.
Bromine-water test The bromine water test is somewhat more satisfactory for pure tryptophane than the glyoxylic acid test. Learn all about bromine water test equation. Basically this experiment checks whether the compounds in the solution are saturated or unsaturated.
Use tubes 2-6 for. Orange Br 2 colorless. Bromine water has a molecular mass of 15981 and a density of 1307 gmL.
Alkenes alkynes Phenols enols Knowns. If bromine water is added to phenylamine the bromine water is decolourised and a white precipitate is formed.
What Is The Chemical Equation Of The Reaction Between Hexane And Bromine Water Quora

Two Step Bromine Attack Feature Rsc Education
What Is The Chemical Equation Of The Reaction Between Hexane And Bromine Water Quora
No comments for "Bromine Water Test Equation"
Post a Comment